For example- ammonia water etc. The atomic orbitals of the same energy level mainly take part in hybridization.

Hybridisation Brilliant Math Science Wiki
Three different types of RNA polymerase exist in eukaryotic.

. It is the mixing of pure atomic orbitals to form equivalent hybrid atomic orbitals. Consensus sequence mutants with wild-type transcripts and they were able to see a decrease in the signal of hybridization. The plan Ive included above is a very small excerpt of the overall floor plan and there are 8 different wall types that have been keyed.
Bonding in many polyatomic compounds cannot be explained by using atomic orbitals. Mendelian inheritance is a type of biological inheritance that follows the principles originally proposed by Gregor Mendel in 1865 and 1866 re-discovered in 1900 by Hugo de Vries and Carl Correns and popularized by William Bateson. These principles were initially controversial.
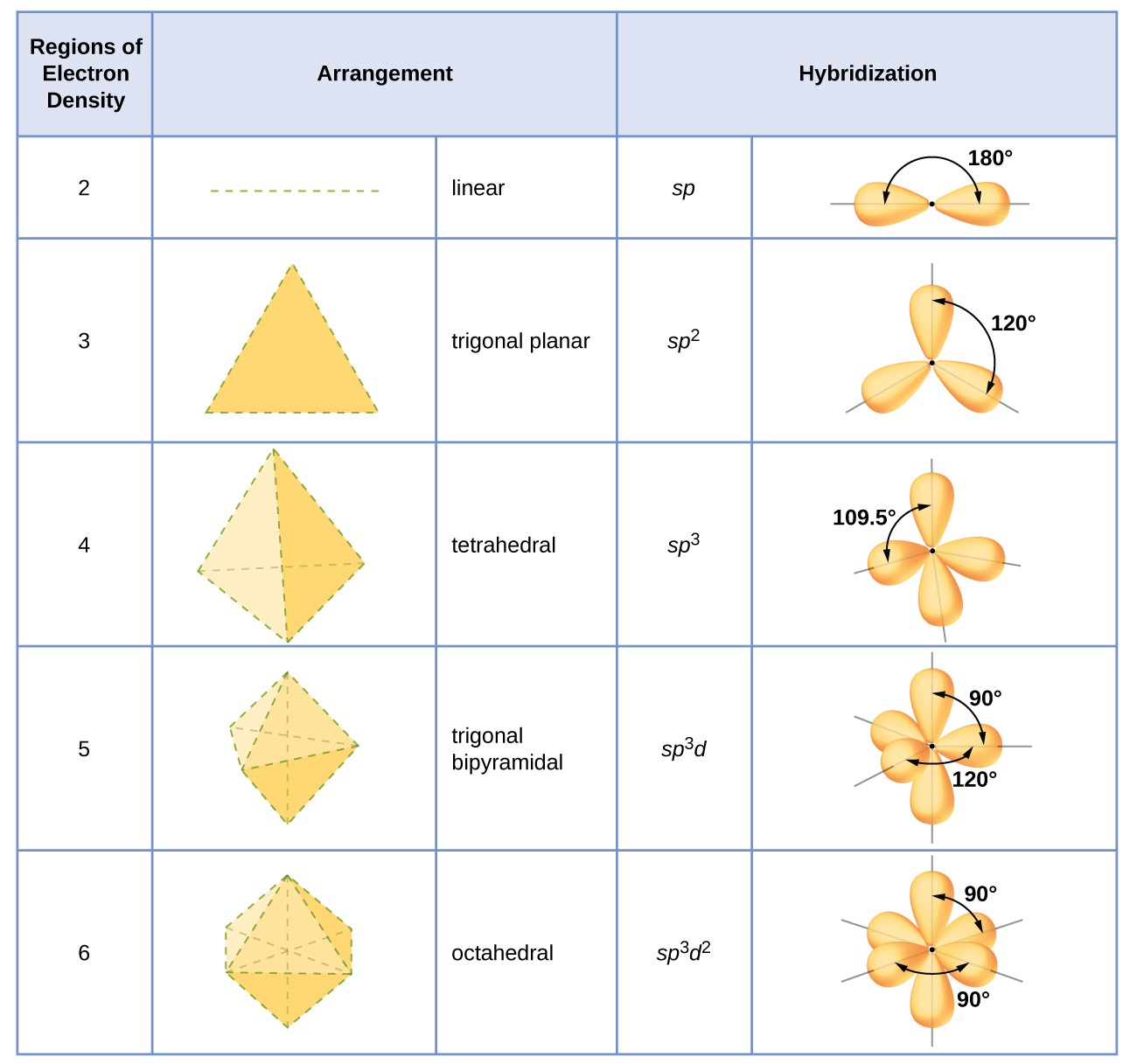
Hybridization in Chemistry is defined as the concept of mixing two atomic orbitals to give rise to a new type of hybridized orbitals. In chemistry orbital hybridisation or hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals with different energies shapes etc than the component atomic orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theoryFor example in a carbon atom which forms four single bonds the valence-shell s orbital combines. This intermixing usually results in the formation of hybrid orbitals having entirely different energies shapes etc.
Check out the hybridization of Ammonia. The concept of hybridization can explain such compounds. And that doesnt include the default wall type a 24 interior wall with 58 gypsum board on each side which is left unkeyed.
When Mendels theories were integrated with the BoveriSutton chromosome theory of inheritance.
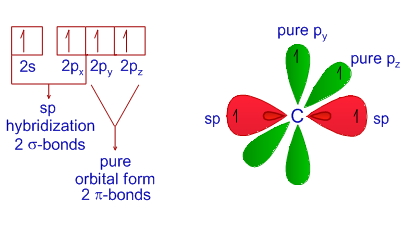
Hybridization Examples In Chemistry Types Sp Sp2 Sp3 Sp3d Sp3d2 Sp3d3 Dsp2

0 Comments